Are you having trouble finding 'how to write an ionic equation'? You will find the answers here.
How to write geographical region equations.STEP 1: Indite the chemical equation.STEP 2: Rewrite aside separating the disintegrable ionic compounds into their dissociated ions.STEP 3: Cancel exterior common ions, which are the spectator pump ions.STEP 4: Compose the net geographic region equation for neutralisation reaction.
Table of contents
- How to write an ionic equation in 2021
- Ionic equation for neutralisation
- Net ionic equation practice
- How to write a net ionic equation
- Complete ionic equation calculator
- Balancing ionic equations
- What is a net ionic equation
- What is an ionic equation
How to write an ionic equation in 2021
 This image representes how to write an ionic equation.
This image representes how to write an ionic equation.
Ionic equation for neutralisation
 This picture shows Ionic equation for neutralisation.
This picture shows Ionic equation for neutralisation.
Net ionic equation practice
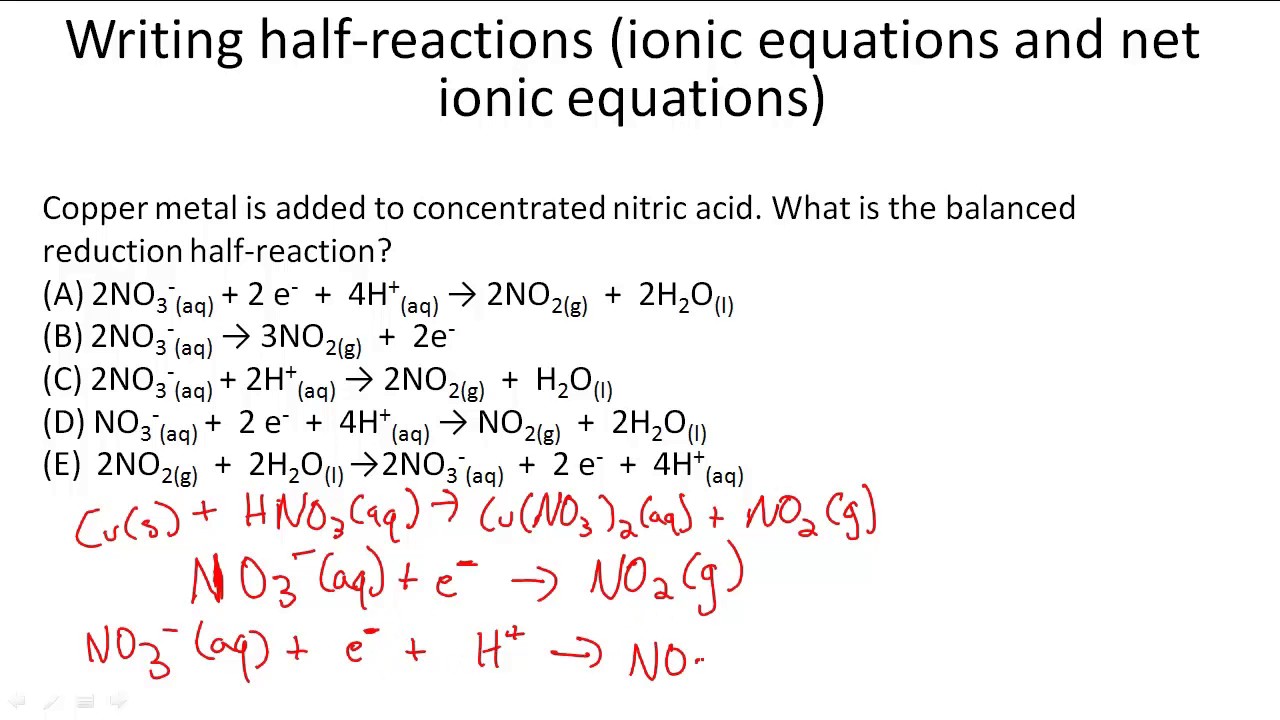 This image shows Net ionic equation practice.
This image shows Net ionic equation practice.
How to write a net ionic equation
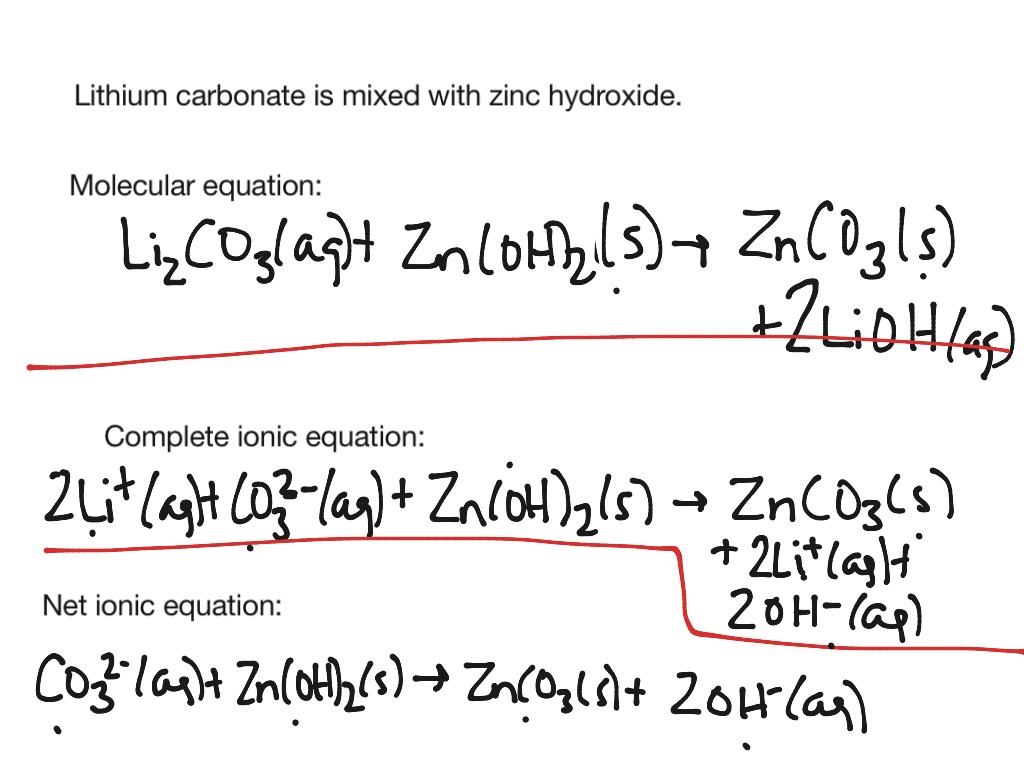 This picture shows How to write a net ionic equation.
This picture shows How to write a net ionic equation.
Complete ionic equation calculator
 This image illustrates Complete ionic equation calculator.
This image illustrates Complete ionic equation calculator.
Balancing ionic equations
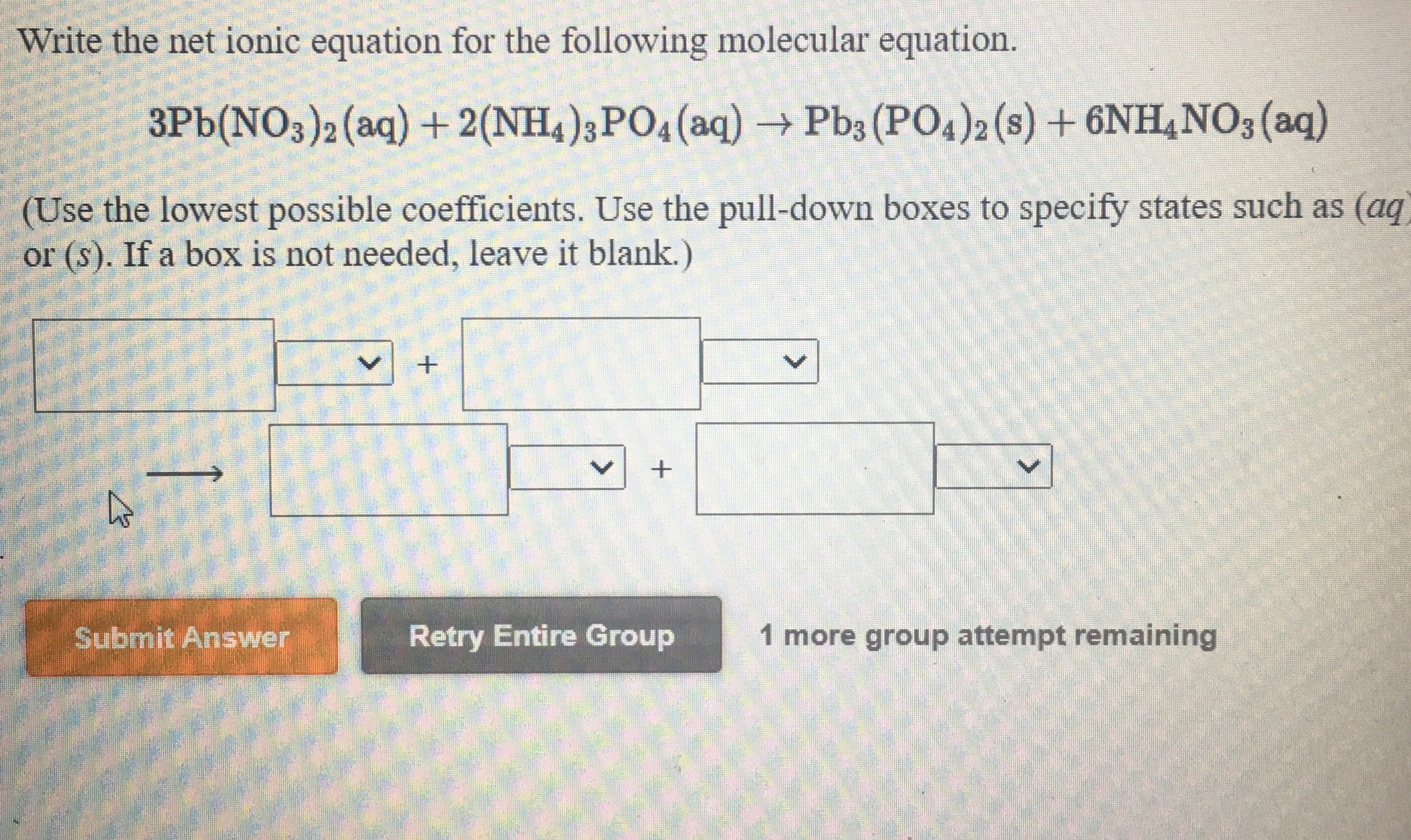 This image representes Balancing ionic equations.
This image representes Balancing ionic equations.
What is a net ionic equation
 This image illustrates What is a net ionic equation.
This image illustrates What is a net ionic equation.
What is an ionic equation
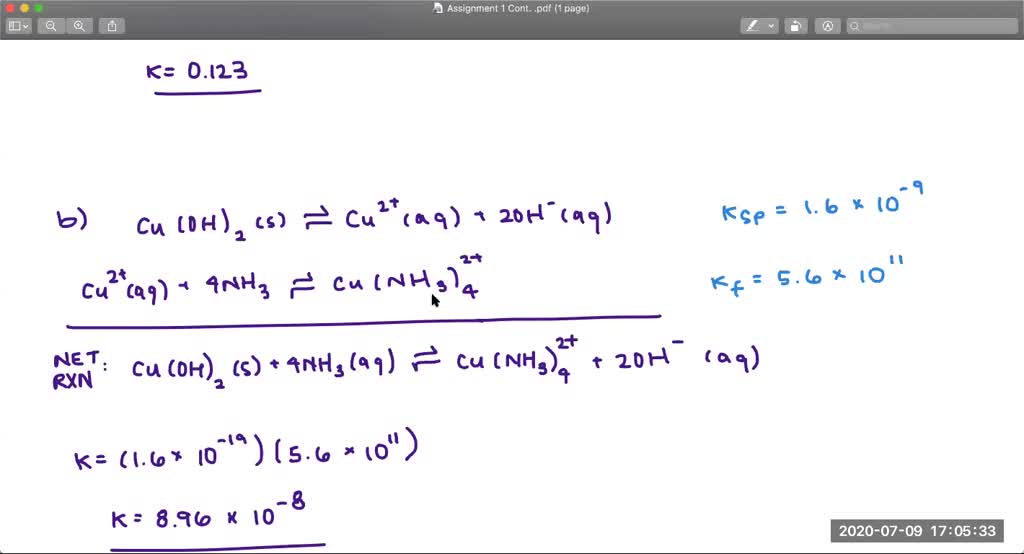 This picture shows What is an ionic equation.
This picture shows What is an ionic equation.
How to write the ionic equation for calcium chloride?
You might have gotten 2H + (aq) + 2OH – (aq) ⟶ 2H 2 O (l). This is technically correct but not simplified to the lowest ratio. Simplify by dividing the coefficients by 2 to get H + (aq) + OH – (aq) ⟶ H 2 O (l). Write the ionic equation for the precipitation reaction between calcium chloride solution and sodium carbonate solution.
How to write an ionic equation for neutralisation?
Ionic Equations for Neutralisation STEP 1: Write the chemical equation HCl (aq) + NaOH (aq) ⟶ NaCl (aq) + H 2 O (l) STEP 2: Rewrite by separating the soluble ionic compounds into their dissociated ions H + (aq) + Cl – (aq) + Na (aq) +... STEP 3: Cancel out common ions, which are the spectator ions H ...
How to write net ionic equation with spectator ions?
Rewrite the action without any of the canceled species. Spectator ions do not participate in the reaction, but they are present. Finishing the example, there are 6Cl - spectator ions on each side that can be canceled out. The final net ionic equation is 2Cr (s) + 3Ni 2+ (aq) --> 2Cr 3+ (aq) + 3Ni (s).
How to write an ionic equation from a word equation?
How To Write An Ionic Equation From A Word Equation? Step 1: Write the equation and balance it if necessary NaCl (aq) + AgNO 3 (aq) → AgCl (s) + NaNO 3 (aq) Step 2: Split the ions. (Only compounds that are aqueous are split into ions.) Step 3: Cancel out spectator ions. (Spectator ions are ions that ...
Last Update: Oct 2021